01.17.11
Meteorology 101: Elements of Weather – Pressure
Last Monday we defined the elements of weather as temperature, pressure, wind, moisture, clouds, precipitation, and visibility. We then took a look at temperature, including what it is and how we define the layers of the atmosphere by temperature (troposphere, stratosphere, mesosphere, and thermosphere).
Today we’re going to look at atmospheric pressure.
————————————————–
Pressure
Pressure is simply defined as force per unit area.
When you sit down on a chair, you are exerting a certain amount of pressure upon the chair due to your weight. Now imagine sitting on a much smaller chair…if the chair is small enough, it’s possible that it might break, as your weight is now distributed across a much smaller surface area (read: the pressure is higher).
Wherever you are on earth, there is a “column” of air above you that is exerting pressure on you (due to the weight of the air molecules). This is what we mean by atmospheric pressure.
Pressure Units
- Pounds per square inch (psi)
- Inches of mercury (in. Hg) – old mercury barometers measured pressure based on how high the mercury rose in a glass tube
- Millibars (mb) or hectoPascals (hPa) – meteorological measurement of pressure (1 mb = 1 hPa)
Ideal Gas Law
While the atmosphere is not entirely made up of ideal gases, the ideal gas law is still a good representation of how air behaves based on the temperature, pressure, and volume. In terms of a column of air sitting above a certain point, the ideal gas law is as follows:
pV = nRT
- p = pressure exerted by air column
- V = volume of air column
- n = number of air molecules in the column
- R = gas constant
- T = temperature of the air column
Changes in Pressure
Based on the ideal gas law, we can make a few assumptions, all of which should be fairly intuitive:
- If the volume goes up, the pressure goes down…think about 20 marbles moving around inside a container; if you put those marbles inside a larger container, and they’re still moving at the same speed, then you would expect there to be less pressure with the larger container
- If the number of molecules goes up, the pressure goes up…now put 20 more marbles inside the container, all moving at the same speed; you should expect the pressure to be higher now
- If the temperature goes up, the pressure goes up…let’s say the marbles speed up, symbolizing a higher temperature; you should expect the pressure to be higher, as the marbles are now hitting the sides of the container more frequently
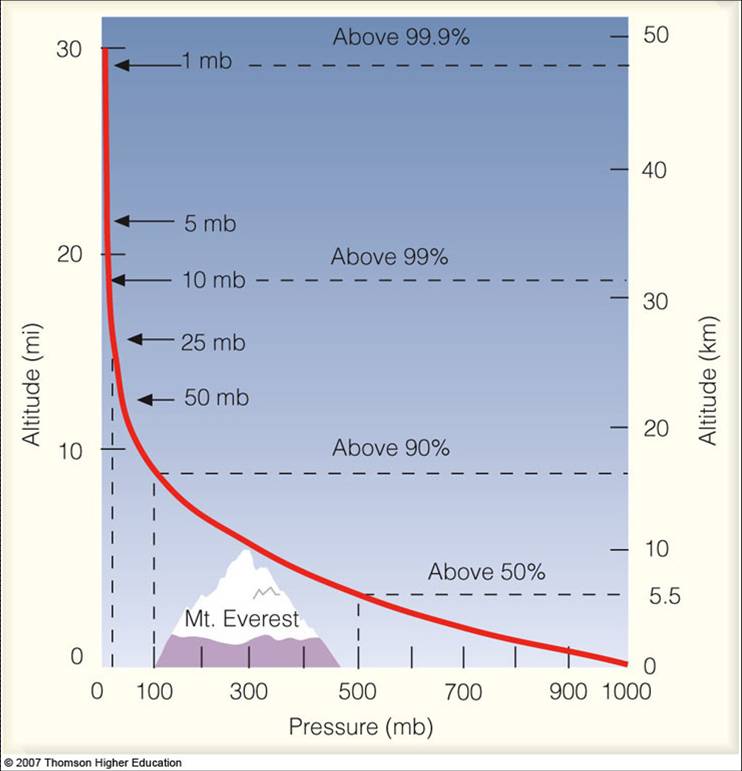
Pressure Structure of the Atmosphere
Meteorologists use pressure as a vertical coordinate (we often use it instead of altitude). As you would expect, pressure decreases as you go up…remember that airplane cabins must be pressurized and mountain climbers must carry oxygen as the air is so thin.
Source: Lyndon State College
Sea-level pressure is 1013 mb. A strong high pressure system might have a pressure of 1040 mb, while a strong low-pressure system might have a pressure of 960 mb (some hurricanes have a pressure as low as 900 mb).
Pressure Corrections
Since the surface pressure at Denver is always going to be lower than the pressure at San Francisco, we do have to make a slight correction for height. We calculate what we call the mean sea-level pressure (MSLP) at every location, so we sort-of level the ground to calculate what the pressure would be at that location if there were no mountains (otherwise, it would look like there is always a low-pressure system over the mountains).
————————————————–
Come back next Monday to learn about the wind, another element of weather related to pressure.